Rajca Research Group
Organic, Polymer and Biomaterials Chemistry
Rational Designs | Syntheses | Measurements


We reported preparation and magnetic studies of the first organic polymer with magnetic ordering at temperature of about 10 K. Because the polymer is based upon relatively reactive triarylmethyl radicals, it has to be handled below 170 K in the absence of oxygen. Two major benchmarks must be attained before polymer-based magnets can be made into practical materials: (1) stability at ambient conditions and (2) magnetic ordering above room temperature. Based upon the body of experimental knowledge on ambient stable S = 1 organic diradicals (with S = 1/S = 0 energy gaps corresponding to temperature of more than 300 K), both targets should be attainable. Following the design principles developed for the polyarylmethyls, our aim is the search for stable polyradicals that are promising building blocks for preparation of organic polymer magnets ("plastic magnets") and for biomedical applications.
Among high-spin di- and poly-radicals that are stable at ambient conditions, numerous S = 1 nitroxide diradicals have been prepared, but only a few of S = 3/2 nitroxide triradicals are known. In 1999, we reported preparation and magnetic properties of the 1,3-phenylene-based nitroxide diradical, in which the para/ortho positions are protected with trifluoromethyl groups. Such steric shielding provided the diradical that is stable at ambient conditions (in the solid state and solution) and stable under the typical normal phase chromatographic conditions (Chem. Commun, 1999, 1249).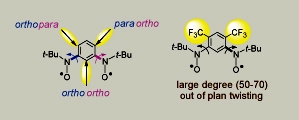 However, steric hindrance of the trifluoromethyl groups causes significant twisting of the nitroxide moieties out of plane of the 1,3-phenylene, with dihedral angles in the 50 - 70° range, as determined by single crystal X-ray structure. This twisting disrupts the π-overlap, weakening exchange coupling between unpaired electrons. Consequently, the singlet-triplet energy gap (ΔEST) between the triplet (S = 1) ground state and the lowest singlet (S = 0) excited state is only about 0.16 kcal/mol. At ambient temperature, the ΔEST is significantly smaller than the thermal energy (0.6 kcal/mol), leading to nearly statistical distribution of diradicals in their S = 1 and S = 0 states; that is, at ambient temperature, such diradical may be viewed as ensemble of two nearly independent S = 1/2 radicals.
However, steric hindrance of the trifluoromethyl groups causes significant twisting of the nitroxide moieties out of plane of the 1,3-phenylene, with dihedral angles in the 50 - 70° range, as determined by single crystal X-ray structure. This twisting disrupts the π-overlap, weakening exchange coupling between unpaired electrons. Consequently, the singlet-triplet energy gap (ΔEST) between the triplet (S = 1) ground state and the lowest singlet (S = 0) excited state is only about 0.16 kcal/mol. At ambient temperature, the ΔEST is significantly smaller than the thermal energy (0.6 kcal/mol), leading to nearly statistical distribution of diradicals in their S = 1 and S = 0 states; that is, at ambient temperature, such diradical may be viewed as ensemble of two nearly independent S = 1/2 radicals.
Diradicals with both stability and strong paramagnetism at ambient conditions may be facilitated by conformationally-restricted structures, in which the radical moieties are co-planar with 1,3-phenylene and are sterically shielded, including the para/ortho positions. The required co-planar structures may be best attained through 6-membered ring annelation of radical moieties to 1,3-phenylene; such conformationally-restricted structures should allow for accommodation of relatively bulky groups to provide adequate steric shielding.
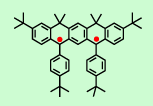 In 1992, our group prepared the annelated S = 1 triarylmethyl-based diradical, which is stable at ambient temperature, but it reacts with atmospheric oxygen. The detailed EPR and magnetic studies of the annelated diradical indicate the S = 1 ground state with ΔEST far exceeding 0.6 kcal/mol (J. Org. Chem., 1992, 57, 1760). Such annelated diradicals are promising building blocks for preparation of organic polymer magnets.
In 1992, our group prepared the annelated S = 1 triarylmethyl-based diradical, which is stable at ambient temperature, but it reacts with atmospheric oxygen. The detailed EPR and magnetic studies of the annelated diradical indicate the S = 1 ground state with ΔEST far exceeding 0.6 kcal/mol (J. Org. Chem., 1992, 57, 1760). Such annelated diradicals are promising building blocks for preparation of organic polymer magnets.
In 2007, we reported the high-spin aminyl diradical, an analogue of the annelated S = 1 triarylmethyl-based diradical (J. Am. Chem. Soc., 2007, 129, 7232). The aminyl diradical is stable in solution at low temperatures and it does not react with oxygen. Magnetic studies by EPR spectroscopy and SQUID magnetometry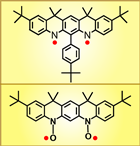 indicate that the diradical is planar and it possesses triplet ground state, with strong ferromagnetic coupling. We have prepared an annelated nitroxide diradical, analogue of the S = 1 aminyl diradical (Chem. Commun., 2009, 4372). This is the first isolated nitroxide diradical with two diarylnitroxide moieties. Both EPR and magnetic studies confirm the S = 1 ground state with ΔEST far exceeding 0.6 kcal/mol. The annelated nitroxide diradical is stable at ambient conditions, in the solid state and in solution.
indicate that the diradical is planar and it possesses triplet ground state, with strong ferromagnetic coupling. We have prepared an annelated nitroxide diradical, analogue of the S = 1 aminyl diradical (Chem. Commun., 2009, 4372). This is the first isolated nitroxide diradical with two diarylnitroxide moieties. Both EPR and magnetic studies confirm the S = 1 ground state with ΔEST far exceeding 0.6 kcal/mol. The annelated nitroxide diradical is stable at ambient conditions, in the solid state and in solution.
We reported synthesis and characterization of aza[1n]metacyclophanes, n = 4, 6, 8, 10, which are potential precursors for macrocyclic high spin polyradicals![aza[1n]metacyclophanes](img/azacyclophane.gif) (J. Org. Chem., 2008, 73, 27). We developed a low temperature method in which the progress of the oxidation of secondary diarylamines with dimethyldioxirane was monitored by EPR spectroscopy and SQUID magnetometry. Using this method, we were able to generate the first m-phenylene-based diaryl
(J. Org. Chem., 2008, 73, 27). We developed a low temperature method in which the progress of the oxidation of secondary diarylamines with dimethyldioxirane was monitored by EPR spectroscopy and SQUID magnetometry. Using this method, we were able to generate the first m-phenylene-based diaryl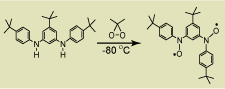 nitroxide diradical, and to determine its triplet ground state for the predominant conformer. However, only high-spin diradicals were detected when this method was applied to oxidation of aza[14]metacyclophane (J. Am. Chem. Soc., 2008, 130, 9099).
nitroxide diradical, and to determine its triplet ground state for the predominant conformer. However, only high-spin diradicals were detected when this method was applied to oxidation of aza[14]metacyclophane (J. Am. Chem. Soc., 2008, 130, 9099).
We prepared and studied another class of high-spin nitroxide diradicals with conformationally-restricted structures, in which nitroxides are annelated to m-phenylene forming tricyclic benzobisoxazine-like structures (J. Am. Chem. Soc., 2007, 129). In 1972, the octamethyl derivative of benzobisoxazine nitroxide diradical was reported to slowly decompose in solution by Rassat and Sieveking, thus its magnetic characterization was incomplete. Intrigued by these results, we set out to investigate further magnetic properties of the diradical and to probe the effect of steric hindrance on stability and magnetic properties of annelated diradicals. We were able to optimize the synthesis and obtained the diradical both in good yield and with high purity. We found that the nitroxide diradical was stable in solution and in solid state. The conformationally-restricted nitroxide moieties are coplanar with the m-phenylene, leading to large values of 2J (2J/k > 200 K in solution and 2J/k 300 K in the solid state). To probe the steric effects, we prepared the diradical in which all ortho and para positions of the m-phenylene are sterically shielded. In this diradical, distortion of the nitroxide moieties from coplanarity is moderate, such that the singlet-triplet gaps remain large in both solution (2J/k > 200 K) and the solid state (2J/k 400-800 K), though an onset of thermal depopulation of the triplet ground state is detectable near room temperature. 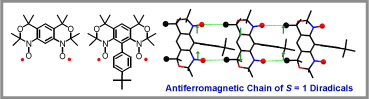 We found that both nitroxide diradicals have robust triplet ground states with strong ferromagnetic coupling and good stability at ambient conditions. Magnetic behavior of the nitroxide diradicals at low temperature is best fit to the model of one-dimensional S = 1 Heisenberg chains with intrachain antiferromagnetic coupling. The antiferromagnetic coupling between the S = 1 diradicals may be associated with the methyl nitroxide C-H- - -O contacts, including nonclassical hydrogen bonds. These unprecedented organic S = 1 antiferromagnetic chains are highly isotropic, compared to those of the extensively studied Ni(II)-based chains.
We found that both nitroxide diradicals have robust triplet ground states with strong ferromagnetic coupling and good stability at ambient conditions. Magnetic behavior of the nitroxide diradicals at low temperature is best fit to the model of one-dimensional S = 1 Heisenberg chains with intrachain antiferromagnetic coupling. The antiferromagnetic coupling between the S = 1 diradicals may be associated with the methyl nitroxide C-H- - -O contacts, including nonclassical hydrogen bonds. These unprecedented organic S = 1 antiferromagnetic chains are highly isotropic, compared to those of the extensively studied Ni(II)-based chains.
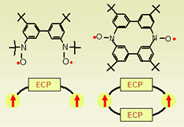 Another project involves preparation of stable nitroxide diradicals with parallel Exchange Coupling Pathways (ECP). The aim is to carried out detailed study on the finding, which we reported earlier (Chem.Comm., 2000, 1021), that singlet-triplet energy gap in the cyclophane-based triarylmethyl diradical with two parallel ECPs is double when compared to the diradical with one ECP. A stable nitroxide diradical with one ECP has been synthesized and studied (see report and poster by Joshua Schmidt, REU student). Preparation of a cyclophane nitroxide diradical (diazacyclophane) with two ECPs is in progress.
Another project involves preparation of stable nitroxide diradicals with parallel Exchange Coupling Pathways (ECP). The aim is to carried out detailed study on the finding, which we reported earlier (Chem.Comm., 2000, 1021), that singlet-triplet energy gap in the cyclophane-based triarylmethyl diradical with two parallel ECPs is double when compared to the diradical with one ECP. A stable nitroxide diradical with one ECP has been synthesized and studied (see report and poster by Joshua Schmidt, REU student). Preparation of a cyclophane nitroxide diradical (diazacyclophane) with two ECPs is in progress.
We have prepared stable calix[4]arene nitroxide raradicals with fixed cone and 1,3 alternate conformations. The nitroxide diradical and tetraradical with fixed cone conformation were reported in 2003 (JACS, 2003, 125, 8534 ). In solution, the calixarene tetraradical has a tetra-fold symmetric conformation on the NMR time scale and a small, but non-negligible, exchange interactions between the radicals (30 K > |J/k| >>1.8 mK). In the solid state, dimerization of one diagonal pair of nitroxides leads to a pinched cone conformation [X-ray structure].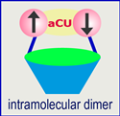 In this intramolecular dimer, the nitroxides have a strong antiferromagnetic interaction, with the coupling constant of |J/k| = 200 - 300 K (singlet-triplet energy gap, ΔEST ≈ 1 kcal/mol). Such intramolecular dimers of stable di- and polyradicals, with conformationally restricted macrocyclic backbone, may provide novel antiferromagnetic coupling units (aCUs), which are essential for effective approaches to polymer-based organic magnets.
In this intramolecular dimer, the nitroxides have a strong antiferromagnetic interaction, with the coupling constant of |J/k| = 200 - 300 K (singlet-triplet energy gap, ΔEST ≈ 1 kcal/mol). Such intramolecular dimers of stable di- and polyradicals, with conformationally restricted macrocyclic backbone, may provide novel antiferromagnetic coupling units (aCUs), which are essential for effective approaches to polymer-based organic magnets.
We prepared the calixarene nitroxide radicals with 1,3 alternate conformations and reinvestigated magnetic properties of the nitroxide radicals with fixed cone conformation (JACS, 2006, 128, 13497). The 1,3-alternate nitroxide diradical and tetraradical provide unique polyradical scaffolds for dissection of the through-bond and through-space intramolecular exchange couplings. Detailed magnetic studies of the calix[4]arene nitroxide tetraradical with fixed cone conformation in solution indicate conformational dependence of exchange coupling. Through-bond coupling between the adjacent nitroxide radicals is mediated by the nitroxide-m-phenylene-CH2-m-phenylene-nitroxide coupling pathway, and through-space coupling is found between the diagonal nitroxide radicals at the conformationally constrained N···N distance of 5-6 Å.
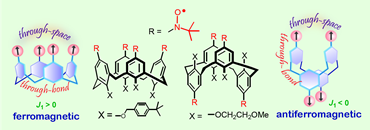 Magnetic studies of the calix[4]arene polyradical scaffolds in frozen solutions show that the through-bond exchange coupling in the 1,3-alternate calix[4]arene tetraradical is antiferromagnetic, while that in cone calix[4]arene tetraradical is ferromagnetic. The through-space exchange couplings are antiferromagnetic in both cone and 1,3-alternate calix[4]arene tetraradical, as well as in the 1,3-alternate calix[4]arene diradical. The exchange coupling constants (J/k) are of the order of 1 K.
Magnetic studies of the calix[4]arene polyradical scaffolds in frozen solutions show that the through-bond exchange coupling in the 1,3-alternate calix[4]arene tetraradical is antiferromagnetic, while that in cone calix[4]arene tetraradical is ferromagnetic. The through-space exchange couplings are antiferromagnetic in both cone and 1,3-alternate calix[4]arene tetraradical, as well as in the 1,3-alternate calix[4]arene diradical. The exchange coupling constants (J/k) are of the order of 1 K.
We also investigated the calix[4]arene nitronyl nitroxide raradicals with 1,3 alternate conformations (Tetrahedron, 2007). In solution, the exchange coupling constants (|J/k|) are much larger than the 14N-hyperfine splitting for nitronyl nitroxides but they are much smaller than those determined for the corresponding nitroxide radicals. Weak antiferromagnetic coupling, that is stronger than the one in solution, is found between neighboring molecules in solidstate. Such 1,3-alternate calix[4]arene nitronyl nitroxide tetraradical and diradical provide a model system for the study of electron spin relaxation in the presence of weak radical-radical interactions.
Since nitroxide radicals (S = 1/2) have a broad biological and medical applications (e.g. spin probes, antioxidants, contrast agents), we ponder the roles of high-spin nitroxide di- and polyradicals (S > 1/2) in such applications. As starting point,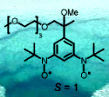 we prepared and studied a triplet ground state (S = 1) pegylated nitroxide diradical, including the effect of water on its magnetic properties (Chem. Comm., 2005, 5047). Also, a glucamide-functionalize nitroxide diradical was prepared and its magnetic properties were investigated. We developed a modular and efficient methodology for the synthesis of tricyclic benzobisoxazines, which are precursors to the nitroxide diradicals with conformationally-restricted structures (J. Org. Chem., 2007, 72, 1867). Our synthetic methodology provides access benzobisoxazines functionalized with various water solubilizing groups as well as to the non-symmetrical diamines (derived from two different ketones). Electron spin relaxation mechanism of selected nitroxide radicals have been studied, in collaboration with Professors Gareth and Sandra Eaton of the Center for EPR Imaging In-Vivo Physiology at the University of Denver (J. Phys. Chem. B, 2008, 112, 2818).
we prepared and studied a triplet ground state (S = 1) pegylated nitroxide diradical, including the effect of water on its magnetic properties (Chem. Comm., 2005, 5047). Also, a glucamide-functionalize nitroxide diradical was prepared and its magnetic properties were investigated. We developed a modular and efficient methodology for the synthesis of tricyclic benzobisoxazines, which are precursors to the nitroxide diradicals with conformationally-restricted structures (J. Org. Chem., 2007, 72, 1867). Our synthetic methodology provides access benzobisoxazines functionalized with various water solubilizing groups as well as to the non-symmetrical diamines (derived from two different ketones). Electron spin relaxation mechanism of selected nitroxide radicals have been studied, in collaboration with Professors Gareth and Sandra Eaton of the Center for EPR Imaging In-Vivo Physiology at the University of Denver (J. Phys. Chem. B, 2008, 112, 2818).
Our interest in this reseach area born out of the collaboration with Professors Gareth and Sandra Eaton to 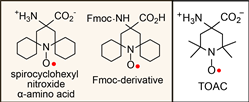 improve the spin-labeling/pulsed EPR technique, which is one of the best methods for accurate measurement of conformational changes and characterization of flexible regions of biomacromolecules. We reported the synthesis and characterization of spirocyclohexyl nitroxide α-amino acid and its N-(9-fluorenylmethoxycarbonyloxy) (Fmoc) derivative (Chem. Eur. J., 2010, 16, 5778).
improve the spin-labeling/pulsed EPR technique, which is one of the best methods for accurate measurement of conformational changes and characterization of flexible regions of biomacromolecules. We reported the synthesis and characterization of spirocyclohexyl nitroxide α-amino acid and its N-(9-fluorenylmethoxycarbonyloxy) (Fmoc) derivative (Chem. Eur. J., 2010, 16, 5778).
Our interest expanded to synthesis of nitroxides with increased stability in vivo, including the development of ORCAs for MRI and spin labels for improved distance measurements by pulsed EPR technique.