Rajca Research Group
Organic, Polymer and Biomaterials Chemistry
Rational Designs | Syntheses | Measurements


Nitroxides are widely used as spin labels for study of biomolecules. Site-directed spin labeling (SDSL) pulsed electron paramagnetic resonance (EPR) is among the best techniques for determination of conformational changes and for characterization of the flexible regions of macromolecules. In a typical approach, doubly-labeled macromolecules are prepared by site-directed spin labeling and a set of distances between spin labels is measured using pulsed electron paramagnetic resonance (EPR). The temperatures at which these measurements can be made are limited by the dynamic averaging effects associated with methyl group rotation in the conventional nitroxide spin label, and therefore have to be performed at about or less than 50 – 65 K, requiring use of liquid helium and involve rigorous experimental setup.
Our goals are to design and synthesize ultra-rigid, small-sized spin labels that would enable accurate, very long distance measurements by pulsed EPR at temperatures significantly above 77 K (boiling point of liquid nitrogen) and ultimately at physiological temperatures.
We reported the synthesis and characterization of spirocyclohexyl nitroxide α-amino acid and its N-(9-fluorenylmethoxycarbonyloxy) (Fmoc) derivative 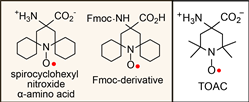 (Chem. Euro. J., 2010). The spirocyclohexyl spin label with rigid structure is an analogue of 2,2,6,6-tetramethylpiperidine-1-oxyl-4-amino-4carboxylic acid (TOAC), the probe of choice for the study of proteins and peptides. We found that replacecing the two methyl groups in TOAC with rigid, spirocyclic cylohexane rings provided the spin label with long phase memory relaxation time that enables the distance measurements by EPR at liquid nitrogen temperature. Attempts to incorporate the Fmoc derivative of spirocyclohexyl spin label into peptides have been unsuccessful so far.
(Chem. Euro. J., 2010). The spirocyclohexyl spin label with rigid structure is an analogue of 2,2,6,6-tetramethylpiperidine-1-oxyl-4-amino-4carboxylic acid (TOAC), the probe of choice for the study of proteins and peptides. We found that replacecing the two methyl groups in TOAC with rigid, spirocyclic cylohexane rings provided the spin label with long phase memory relaxation time that enables the distance measurements by EPR at liquid nitrogen temperature. Attempts to incorporate the Fmoc derivative of spirocyclohexyl spin label into peptides have been unsuccessful so far.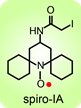 We prepared another derivative, spirocyclohexyl iodoacetamide (spiro-IA) spin label, which bind covalently with the thiol group of a cysteine residue. In collaboration with Professor Hassane Mchaourab at Vanderbilt University, spiro-IA labels have been successfully incorporated into T4 lysozyme (T4L) by SDSL. DEER measurements at room temperature have been successfully performed on the spin labeled T4L in a disaccharide (trehalose) glassy matrix at University of Denver in the Eaton laboratory (Biophys. J., 2015).
We prepared another derivative, spirocyclohexyl iodoacetamide (spiro-IA) spin label, which bind covalently with the thiol group of a cysteine residue. In collaboration with Professor Hassane Mchaourab at Vanderbilt University, spiro-IA labels have been successfully incorporated into T4 lysozyme (T4L) by SDSL. DEER measurements at room temperature have been successfully performed on the spin labeled T4L in a disaccharide (trehalose) glassy matrix at University of Denver in the Eaton laboratory (Biophys. J., 2015).
As a team member of the Protein Core at Membrane Protein Structural Dynamics Consortium (MPSDC), we lend expertise in organic radical chemistry and synthesis to the development of new protein expression protocols.
The spin labelling of proteins by incorporating unnatural amino acid functionalized with nitroxides has many potential advantages over the established, site-directed spin labeling (SDSL) methods. However, despite the tremendous promise of unnatural amino acid spin labels for genetic encoding strategies, a major challenge remains in the survival of such probes in the reducing conditions during the ribosome-mediated protein synthesis, 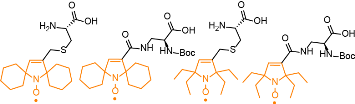 resulting in irreversible chemical reduction of the nitroxide to the corresponding diamagnetic hydroxylamine. We recently synthesized a series of unnatural amino acid spin labels that are resistant to reduction by ascorbate (Org. Lett., 10014). These labels have great potential for enabling the structure-function study of expressed proteins in biological environments.
resulting in irreversible chemical reduction of the nitroxide to the corresponding diamagnetic hydroxylamine. We recently synthesized a series of unnatural amino acid spin labels that are resistant to reduction by ascorbate (Org. Lett., 10014). These labels have great potential for enabling the structure-function study of expressed proteins in biological environments.