Rajca Research Group
Organic, Polymer and Biomaterials Chemistry
Rational Designs | Syntheses | Measurements


Nuclear magnetic resonance imaging (MRI) is one of the most powerful, non-invasive imaging tools for clinical diagnosis and screening. Paramagnetic materials are oftenly used in contrast-enhanced MRI scans to provide improved sensitivity and specificity. All MRI contrast agents currently in clinical use are metal-based, with gadolinium-based contrast agents (GBCAs) being the most widely used. Although most GBCAs are safe for the majority of patients, patients with renal insufficiency are at an increased risk for developing a serious systemic fibrosing disease (NSF) after receiving a GBCA [FDA Safety Warnings | FDA Questions and Answers on GBCAs]
Our goal is to develop of biostable organic radicals as contrast agent for MRI. Metal-free, organic-based agents would avoid the risk associated with toxic metal ions and provide an alternative to conventional paramagnetic agents.
The long-standing obstacle in the development of a practical organic radical contrast agent (ORCA) for MRI is the design and synthesis of paramagnetic organic compounds of moderate molecular size that possess sufficiently long in vivo lifetime, high water relaxivity, and high water solubility.
We synthesized spirocyclohexyl nitroxide that may be viewed as sterically-shielded analogues of the commonly-used nitroxide, 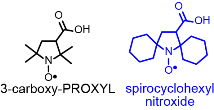 3-carboxy-PROXYL, and found that it was reduced by ascorbate at a significantly slower rate. Note that 3-carboxy-PROXYL is among the most widely used nitroxides and perhaps the most resistant to reduction, with half-life (reduction plus excretion) of 100 ± 50 sec in bloodstream, as determined by MRI mouse studies. This result prompted us to explore the novel molecular design and synthesis ORCAs.
3-carboxy-PROXYL, and found that it was reduced by ascorbate at a significantly slower rate. Note that 3-carboxy-PROXYL is among the most widely used nitroxides and perhaps the most resistant to reduction, with half-life (reduction plus excretion) of 100 ± 50 sec in bloodstream, as determined by MRI mouse studies. This result prompted us to explore the novel molecular design and synthesis ORCAs.
Typical nitroxid radicals with one unpaired electron possess low water relaxivity. Connecting nitroxides to rigid macromolecules such as polymers, proteins and particles can improve the molecular relaxivity. There are many examples of macromolecular conjugated nitroxides, including dendrimers, but these developments have yet to provide a practical clinical agent for MRI. Dendrimers have been demonstrated to be more effective as scaffold in the approaches to increased relaxivity of GBCAs, and therefore they are the scaffold of choice in our design of ORCAs. However, because hydrophobicity of nitroxide-covered dendrimer surfaces may contribute to limited applications of the agents in MRI, our approach is aimed at alleviating the effect by PEGylation. That is, after a fraction of the dendrimer termini are conjugated with nitroxides, the dendrimer will be fully conjugated with polyethylene glycol chains (PEGs). The non-immunogenic and polar PEGs may provide additional advantages, by immobilizing nitroxides as well as increase water access to the paramagnetic nitroxides that could lead to increase molecular relaxivity.
Our success in preparation of spirocyclohexyl nitroxide radicals that are highly resistant to reduction puts us in a unique position in the development of ORCAs. This allowed us to test our molecular design to alleviate hydrophobicity of the rigid scaffold surface, one of the factors limiting application of the current nitroxide-dendrimer agents, using PEGylation technology.
We successfully prepared an i.v injectable ORCA, 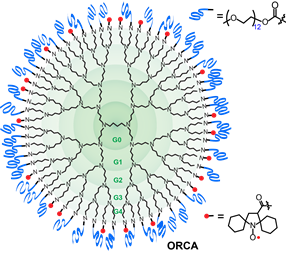 based on spirocyclohexyl nitroxide radicals and polyethylene glycol (PEG) chains conjugated to a generation 4 polypropylenimine (PPI) dendrimers scaffold, that has a long shelf‐life and provides selectively enhanced MRI in mice for over 1 h (J. Am. Chem. Soc., 2012). The large PPI-G4 allowed flexibility in adjusting the ratio of mPEGs and nitroxide to obtain the first ORCA that has a long shelf‐life and provides enhanced MRI in mice for over 1 h. The results demonstrate that ORCA can be designed to have high relaxivity to provide high resolution images of mouse organs. Molecular size of the ORCA based on PPI-G4 is at the upper size-limit for passage through the renal glomerular barrier, undergoes slow excretion through the kidneys. Optimization of ORCAs to acheive high water relaxivity, high water solubility and small, compact molecular size is the next crucial step in the development of ORCAs for clinical application.
based on spirocyclohexyl nitroxide radicals and polyethylene glycol (PEG) chains conjugated to a generation 4 polypropylenimine (PPI) dendrimers scaffold, that has a long shelf‐life and provides selectively enhanced MRI in mice for over 1 h (J. Am. Chem. Soc., 2012). The large PPI-G4 allowed flexibility in adjusting the ratio of mPEGs and nitroxide to obtain the first ORCA that has a long shelf‐life and provides enhanced MRI in mice for over 1 h. The results demonstrate that ORCA can be designed to have high relaxivity to provide high resolution images of mouse organs. Molecular size of the ORCA based on PPI-G4 is at the upper size-limit for passage through the renal glomerular barrier, undergoes slow excretion through the kidneys. Optimization of ORCAs to acheive high water relaxivity, high water solubility and small, compact molecular size is the next crucial step in the development of ORCAs for clinical application.
Molecular relaxivity of the ORCA based on PPI-G4 is ≈ 5 mM-1s-1, comparable to that of the clinical GBCAs. According to the theoretical model of water relaxivity, it will be possible to attain increased molecular relaxivity. 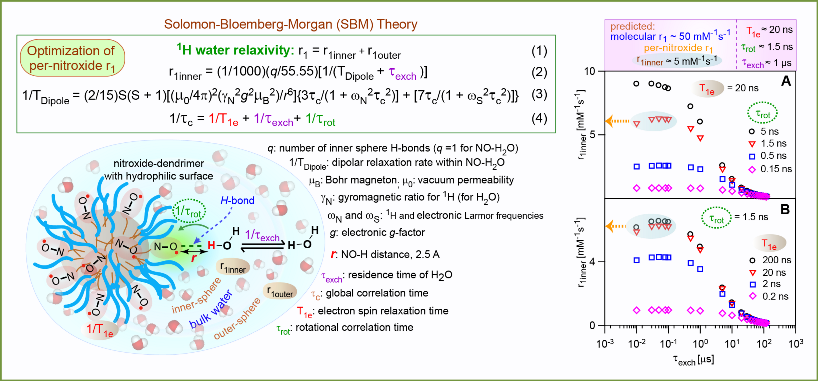
 The ORCAs with high water relaxivity and compact molecular sizes may be attained by increasing density of nitroxides and hydrohilicity at the surface of small dendimer scaffold. We have devised strategies to obtain the target ORCAs, including prepation of tetraethyl nitroxide that is reduced by ascorbate or ascorbate/glutathione at the slowest rate among known nitroxides (Org. Lett., 2012). We are eager to move forward this exciting project when funding is available.
The ORCAs with high water relaxivity and compact molecular sizes may be attained by increasing density of nitroxides and hydrohilicity at the surface of small dendimer scaffold. We have devised strategies to obtain the target ORCAs, including prepation of tetraethyl nitroxide that is reduced by ascorbate or ascorbate/glutathione at the slowest rate among known nitroxides (Org. Lett., 2012). We are eager to move forward this exciting project when funding is available.
In collaboration with the Johnson Research Group at MIT, we investigate another class of ORCAs based on polymer brush nanostructures, to explore functional benefits and advantages of the ORCAs. The Johnson lab designed a new class of branched-bottlebrush polymer dual-modality organic radical contrast agents —ORCAFluors— for combined magnetic resonance and near-infrared fluorescence imaging in vivo (Nat. Commun., 1014, MIT News), NIBIB Science Highlight.
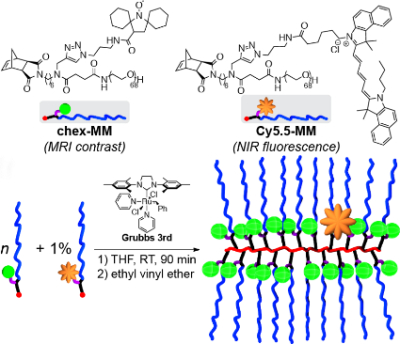 The polymer nanoparticles incorporating spirocyclohexyl nitroxide display a unique compensatory redox response. In the native state, fluorescence is partially quenched by surrounding nitroxides and upon exposure to ascorbate or ascorbate/glutathione that leads to nitroxide reduction, fluorescence emission increases 2- to 3.5-fold (in vitro). This behavior enables correlation of MRI contrast, ex vivo fluorescence intensity and EPR spin concentration with tissues known to possess high concentrations of ascorbate in mice such as liver and brain. The in vitro, in vivo, and ex vivo results, along with modular synthetic approach, make ORCAFluors a promising new platform for multimodality molecular imaging. However, ORCAFluors possess relatively low per nitroxide r1 ~ 0.3 mM-1s-1 at 7 T and relatively large polymer nanoparticle (rSE ~ 10 nm), which is well above the size limit for glomerular filtration. This collaborative project represents a synergistic intersection between nanostructures developed by Johnson lab and the state-of-the-art dendritic ORCAs developed by Rajca lab.
The polymer nanoparticles incorporating spirocyclohexyl nitroxide display a unique compensatory redox response. In the native state, fluorescence is partially quenched by surrounding nitroxides and upon exposure to ascorbate or ascorbate/glutathione that leads to nitroxide reduction, fluorescence emission increases 2- to 3.5-fold (in vitro). This behavior enables correlation of MRI contrast, ex vivo fluorescence intensity and EPR spin concentration with tissues known to possess high concentrations of ascorbate in mice such as liver and brain. The in vitro, in vivo, and ex vivo results, along with modular synthetic approach, make ORCAFluors a promising new platform for multimodality molecular imaging. However, ORCAFluors possess relatively low per nitroxide r1 ~ 0.3 mM-1s-1 at 7 T and relatively large polymer nanoparticle (rSE ~ 10 nm), which is well above the size limit for glomerular filtration. This collaborative project represents a synergistic intersection between nanostructures developed by Johnson lab and the state-of-the-art dendritic ORCAs developed by Rajca lab.