Rajca Research Group
Organic, Polymer and Biomaterials Chemistry
Rational Designs | Syntheses | Measurements


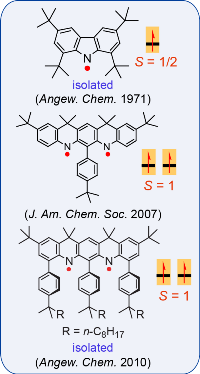 Nitrogen-centered (aminyl) radicals are typically short-lived, detected as intermediates in a variety of chemical and biological processes. Curious by the report on isolation of stable aminyl radical, tetra-tert-butylcarbazole aminyl, by Neugebauer and Fischer in 1971, we explored feasibilty of high spin aminyl radicals. In 2007, we reported the first high spin aminyl diradical that possesses triplet (S = 1) ground state (J. Am. Chem. Soc. 2007). The high spin aminyl is persistant at low temperature (half-life of 30 min at –70 °C). With improved molecular design, we prepared and isolated a triplet ground state aminyl diradical that
Nitrogen-centered (aminyl) radicals are typically short-lived, detected as intermediates in a variety of chemical and biological processes. Curious by the report on isolation of stable aminyl radical, tetra-tert-butylcarbazole aminyl, by Neugebauer and Fischer in 1971, we explored feasibilty of high spin aminyl radicals. In 2007, we reported the first high spin aminyl diradical that possesses triplet (S = 1) ground state (J. Am. Chem. Soc. 2007). The high spin aminyl is persistant at low temperature (half-life of 30 min at –70 °C). With improved molecular design, we prepared and isolated a triplet ground state aminyl diradical that 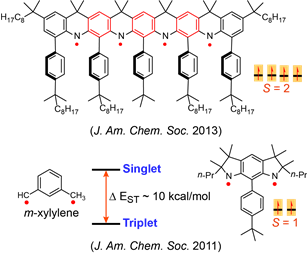 is persistent at room temperature and possesses a singlet-triplet energy gap that is significantly greater than the thermal energy at room temperature (Angew. Chem. Int. Ed. 2010). Recently, we reported high spin aminyl tetraradicals that have quintet (S = 2) ground states and persistent in solution at room temperature (J. Am. Chem. Soc. 2013).
is persistent at room temperature and possesses a singlet-triplet energy gap that is significantly greater than the thermal energy at room temperature (Angew. Chem. Int. Ed. 2010). Recently, we reported high spin aminyl tetraradicals that have quintet (S = 2) ground states and persistent in solution at room temperature (J. Am. Chem. Soc. 2013).
We also prepared another class of aminyl radicals that is a derivative of the well-known reactive intermediate m-xylylene diradical which possesses one of the largest singlet-triplet energy gaps (Δ EST ~ 9.6 kcal/mol) for an organic molecule. The planar derivative of aza-m-xylylene, aminyl diradical possesses a triplet ground state with Δ EST of about 10 kcal/mol. The aminyl diradical persists in solution at room temperature with half-life of 10 minutes (J. Am. Chem. Soc. 2011).
Ongoing research projects have the following specific objectives: (1) aminyl di- and triradicals with very strong pairwise ferromagnetic coupling between electron spins and persistence at room temperature with half-life that would permit isolation of the radicals, (2) aminyl polyradicals polymers with very large magnetic moment. Our long-term goal is a purely organic polymer with very large magnetic moment and magnetic order at room temperature.