Studies of Biological Interactions & Personalized Medicine
Another way in which we use affinity ligands is to study the reactions that take place between proteins and other biological agents. We are well-known in this field and are especially interested in using immobilized proteins as models for the same proteins in humans or in animals. These immobilized proteins are used to create a synthetic system that mimics the interactions of these proteins with drugs or other agents. This makes this approach of great interest for screening possible drug candidates and for predicting the behavior of drug in the body.
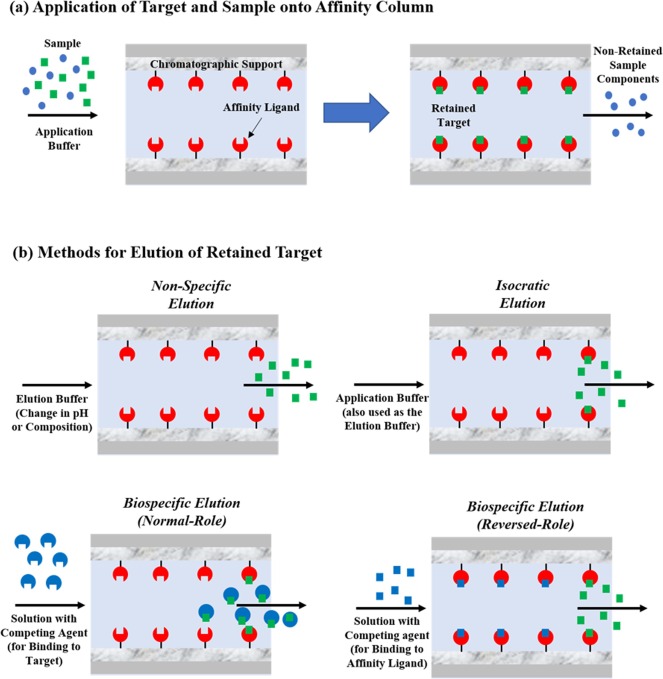
Fig. The (a) sample application/washing steps and (b) examples of elution methods that are used in the on/off mode of affinity chromatography. The three types of elution shown in (b) are non-specific elution, isocratic elution, and biospecific elution. Methods for biospecific elution can be further divided into normal-role elution, in which a competing agent binds to the target, and reversed-role elution, in which the competing agent binds to the immobilized affinity ligand.
One protein we have studied extensively is human serum albumin (HSA). HSA is the most abundant protein in serum and plays an important role in delivering many types of drugs throughout the body. We attach HSA within HPLC columns and routinely use these columns to examine how this protein binds to various drugs, hormones, and other small solutes. Chemicals that we have studied with these columns have included warfarin, thyroxine, tryptophan, ibuprofen, digitoxin, clomiphene, tamoxifen, phenytoin, and carbamazepine. We have also created a similar column based on the serum protein alpha-1 acid glycoprotein (AGP) that can be used to examine the interactions of this protein with various drugs.
As part of this work, we have developed several new approaches for examining the protein binding of drugs and small solutes by high-performance affinity chromatography (HPAC) and affinity capillary electrophoresis (ACE). Examples include approaches we have reported for the study of low solubility compounds, agents with multiple binding sites on a protein, and drugs that have allosteric interactions with a binding protein. In recent work we have also described an ultrafast technique that looks directly at the non-bound fraction of a drug or hormone in a protein sample. This method, which makes use of millisecond-scale separations on an antibody-based column, has great potential as a new tool for the analysis of clinical and pharmaceutical samples.
More recently, we have used HPAC to examine the effects of a disease state such as diabetes on the binding of different solutes to HSA. In particular, a high blood level of glucose in diabetics has proven to cause the non-enzymatic glycation of serum proteins, including HSA. For this purpose, silica columns containing immobilized and glycated HSA has been used to study the interactions of several anti-diabetic agents, such as sulfonylureas, with glycated HSA. We have performed frontal analysis and site-specific competition studies in order to obtain a better quantitative understanding of how glycation can affect drug-protein binding for individuals with diabetes. Ongoing experiments include the use of glycated HSA isolated from known diabetic patients to aid in the development of personalized therapeutic regimes in order to improve the quality of treatment as well as establish dosage margins based on a personal profile.
Examples of Recent Work in Studies of Biological interactions and Personalized Medicine
1.) Elliott L Rodriguez, Pingyang Tao, Ashley G Woolfork, Zhao Li, Ryan Matsuda, Zuchen Sun, David S Hage*, “Studies of binding by sulfonylureas with glyoxal-and methylglyoxal-modified albumin by immunoextraction using affinity microcolumns”, J.Chromatogr. A, 1638 (2021) 461683.
2.) Sandya R Beeram, Chenhua Zhang, Kyungah Suh, William A Clarke, David S Hage*, “Characterization of Drug Binding with Alpha1-Acid Glycoprotein in Clinical Samples using Ultrafast Affinity Extraction”, J.Chromatogr. A, 1649 (2021) 462240.
3.) Susan T Ovbude, Pingyang Tao, Zhao Li, David S Hage*, “High-Performance affinity chromatographic studies of repaglinide and nateglinide interactions with normal and glyoxal-or methylglyoxal-modified human albumin serum”, J. Pharm. Biomed. Anal., 201 (2021) 114097.
4.) Ashley G. Woolfork, Kyungah Suh, Miranda Weigand, David S. Hage*, “Studies of Binding by 2-Imidazolines to Human Serum Albumin and Alpha1-Acid Glycoprotein by High-Performance Affinity Chromatography”, J. Pharm. Biomed. Anal., 202 (2021) 114135.
5.) Chenhua Zhang, Shae Lott, William Clarke and David S. Hage*, “Development of a Microcolumn One-Site Immunometric Assay for a Protein Biomarker: Analysis of Alpha1-Acid Glycoprotein in Serum”, J.Chromatogr. A, 1610 (2020) 460558.
6.) Elliott Rodriguez, Saumen Poddar, Meera Choksi, and David S. Hage*, “Development of an On-line Immunoextraction/Entrapment System for Protein Capture and Use in Drug Binding Studies by High-Performance Affinity Chromatography”, J. Chromatogr. B, 1136 (2020) 121812.
