Affinity Monolith Chromatography
Another area our research group has been a leader in is the creation of a new technique called affinity monolith chromatography (AMC). Our work in this area was featured in the cover article on “Monolithic Chromatography” that appeared in the Dec. 11, 2006, issue of Chemical & Engineering News. AMC is a type of liquid chromatography that combines the use of a monolithic support with a biologically related binding agent as the stationary phase. Monolithic supports are continuous bed supports that have a higher external porosity than particle-based supports, which gives them higher permeability and lower back pressures. Monolithic supports have been gaining in popularity over the last few years in HPLC and have recently also seen growing use in affinity chromatography because of their many advantages. One advantage is their lower back pressures compared to traditional particle-based supports, which can lead to faster separations and shorter analysis times through the ability to operate at higher flow rates. Another advantage is that monolithic supports can be easily modified to attach various ligands for use in HPAC.
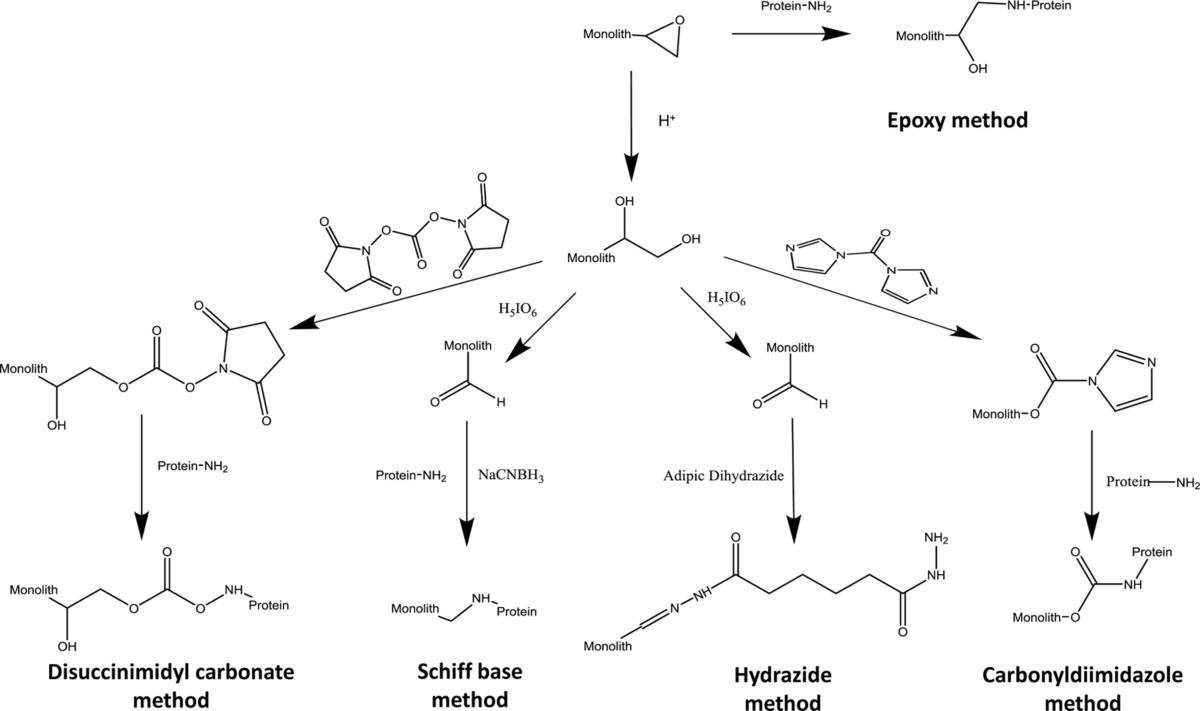
Fig: Examples of common covalent immobilization techniques that have been used in AMC, including the epoxy method, Schiff base method, carbonyldiimidazole method, disuccinimidyl carbonate method, and hydrazide method.
One early study by our group in this field examined the immobilization of antibodies onto a monolith based on a glycidyl methacrylate/ethylene dimethacrylate (GMA/EDMA) co-polymer that we developed for use in immunoaffinity chromatography. This report examined how variations in the polymerization conditions (e.g., porogen composition, polymerization temperature and time, and amount of cross-linking agent) affected the amount of antibodies that could be placed within such a monolith. Several immobilization methods were also explored for use in this work, including the epoxy method, Schiff base, and hydrazide method. Antibodies that were immobilized under optimized conditions were then prepared in small disk columns for use in ultrafast immunoextraction, allowing quantitative binding by the antibodies to a small target within 100 ms (see Figure 1). Other antibodies have since been successfully immobilized onto GMA/EDMA monoliths for other applications, including extractions for capillary electrophoresis and HPLC.
More recent studies by our group have used AMC to perform chiral separations of various drugs by using human serum albumin (HSA) as a stationary phase immobilized to monolithic supports. Many common chiral stationary phases make use of biological binding agents such as carbohydrates or proteins, which are naturally chiral. Various immobilization schemes were compared in this study using GMA/EDMA monoliths, including Schiff base, epoxy, CDI and disuccinimidyl carbonate methods. It was shown that the Schiff base gave the highest protein content and the best chiral separation for mixtures of (R,S)-warfarin and D/L-tryptophan. Two other recent studies by our group used silica monoliths with both HSA and alpha 1-acid glycoprotein (AGP) for chiral separations. An example of such work is given in the Figure 2. It was found that the silica monoliths allowed for greater protein coverage and higher retention than either GMA/EDMA monoliths or particle-based silica columns, thus allowing for better resolution of chiral solutes that could bind to the immobilized proteins in these columns.
Monolithic supports are also being used in our group to study these biological interactions. Two recent studies by students in the Hage laboratory used frontal analysis to study the interaction between carbamazepine and HSA or AGP. Carbamazepine is a drug known to bind to both of these proteins. Silica monoliths containing either HSA or AGP were used in the columns, and various concentrations of carbamazepine were continuously passed through each column. The association equilibrium constants and binding capacities for carbamazepine with these proteins were then determined from these studies.
Yet another application of AMC that we have been researched is the use of this method in the high-throughput analysis of drug-protein interactions. In these work, we have been able to show that silica monoliths can be used in affinity micrcolumns with lengths as little as 1 – 5 mm for binding studies. With these affinity microcolumns, the interaction of drugs with HSA could be measured within seconds, resulting in very low analysis times when compared to silica particle-based supports.
Examples of Recent Work in Affinity Monolith Chromatography
1.) Zhao Li, Elliott Rodriguez, Shiden Azaria, Allegra Pekarek, and David S. Hage*, “Affinity Monolith Chromatography: A Review of General Principles and Applications”, Electrophoresis, 38 (2017) 2837-2850. (invited paper; top downloaded article for Electrophoresis (top 20) – 2017-2018).