Reseach
Click here to view a Slide Show Presentation about Dr. Eckhardt's Research
The role of mechanical energy in chemical processes is being addressed through determining the mechanical properties of energetic materials and pharmaceuticals, both notoriously susceptible to stress-induced chemistry. Of particular interest is how chemical behavior is related to mechanical stress induced either by pressure, defects or light. Understanding requires quantitative measure of the anisotropy of elasticity, the nature and concentration of defects and the photoelasticity of various organic materials. This information is virtually non-existent and is being used to provide a foundation for understanding processes such as detonation and "browning" of pharmaceuticals.
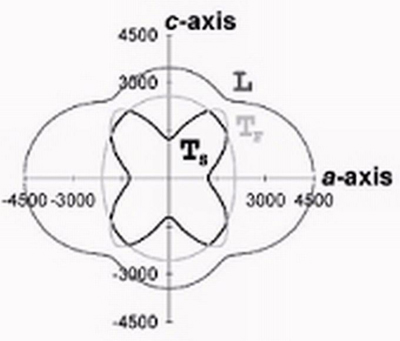
Sound velocity diagram for the ac-plane of the energetic material RDX.
The microscopic origins of friction are being investigated by design of unique amphiphiles that are used to form either Langmuir-Blodgett or SAM films. The surfaces formed by the films have specific chemical modifications that are designed to influence the frictional properties of the films. The nanofriction, measured friction force microscopy, is then compared to macroscopic friction.
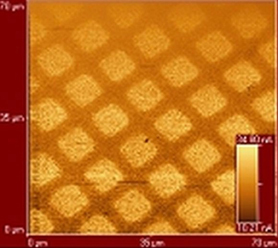
Friction-force microscopy image of two monolayer films with different frictional behavior.
Intermolecular forces are being studied through the guest-host interactions of inclusion compounds. The influence of different guests on the physical properties, e.g. elasticity, heat of fusion, of the host crystals is related to intermolecular interactions.
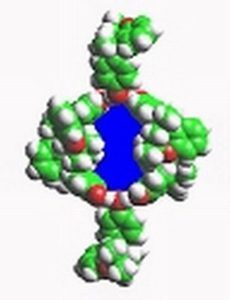
Dianin's inclusion compound showing interior cavity for guests.
Craig J. Eckhardt
Professor
University of Nebraska - Lincoln
Department of Chemistry
524 HAH
Lincoln NE 68588-0304
E-mail: ceckhardt1@unl.edu
Off: (402) 472 2734
Lab: (402) 472 1429
