|

|
|
Department of Chemistry
Hamilton Hall
University of Nebraska
Lincoln, NE 68588-0304
Phone: (402)472-9388
|
|
|
|
 |
 |
| Triradical |
|
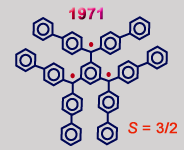
Preparation of organic triradical was first attempted by Martin Leo in 1937, by treating trichloride with Cu. However, the product was not characterized. A slightly more stabilized triradical, 1,3,5-tris(di-p-biphenylmethyl)benzene, was prepared in 1965, but its characterization was not established until 1971. This triradical was prone to rapid dimerization.
|
| |
| References |
- M. Leo, "Uber Radikale mit mehreren dreiwertigen Kohlenstoffatomen", Ber., 1937, 70, 1691.
- G. Schmauss, H. Baumgartel, and H. Zimmermann, "1,3,5-Tris(di-p-biphenylmethyl)benzene, a New Triradical", Angew. Chem. Int. Ed., 1965, 4, 596.
- G. Kothe, E. Ohmes, J.Brickmann, and H. Zimmermann, "1,3,5-Benzenetriyltris[di(p-biphenyl)methyl], a Radical Having a Quartet Ground State that Dimerizes by Entropy", Angew. Chem. Int. Ed., 1971, 10, 938.
- J. Brickmann and G. Kothe, "ESR of the quartet state of randomly oriented molecules: Calculation of the line shape ad detection of the zero-field splitting", J. Chem. Phys., 1973, 59, 2807.
|
|
Our research group also prepared several triradicals. The iso-propyl-substituted triradicals were isolated as solids.

- A. Rajca, S. Utamapanya, "Polyarylmethyl Quartet Triradicals and Quintet Tetraradicals", J. Am. Chem. Soc., 1993, 115, 2396.
- S. Rajca, A. Rajca, "Novel High-Spin Molecules: π-Conjugated Polyradical Polyanions. Ferromagnetic Spin Coupling and Electron Localization", J. Am. Chem. Soc., 1995, 117, 9172.
To see more structures of triradicals prepared by our group, follow this
link.
|
| |
|
|
|
 |
|
 |
|




