|
Our research group prepared several derivatives of Schlenk diradical. To improve stability of the diradical, alkyl groups (e.g. t-Bu, Me, and i-Pr) were introduced at the para position of benzene rings, with respect to the triarylmethyl radical sites.
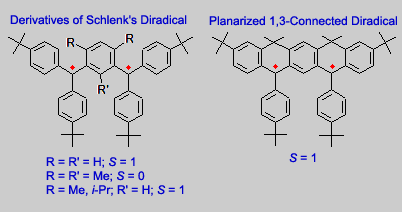
While the diradical with R = R´ = H had to be handled in solution at low temperature (-78 °C or lower), the alkyl-substituted diradicals were quite stable at room temperature, such that they could be isolated as solids.
The diradicals project began about two years after Andrzej Rajca started his research group at Kansas State University (KSU). The diradicals were prepared by the carbanion method, which was just developed for the preparation of the Polyarylmethyl Quintet Tetraradical. This method efficiently produced the alkyl-substituted Schlenk diradicals, with only small amount of monoradical impurity. For the planarized diradical, treatment of the corresponding hydrocarbon precursor with MeLi or KH gave the carbanion, which was oxidized with I2 to give the diradical.
- A. Rajca, S. Utamapanya, J. Xu, "Control of Magnetic Interactions in Polyarylmethyl Triplet Diradicals Using Steric Hindrance", J. Am. Chem. Soc., 1991, 113, 9235.
- S. Utamapanya, A. Rajca, "Topological Control of Electron Localization in π-Conjugated Polyarylmethyl Carbopolyanions and Radical Anions", J. Am. Chem. Soc., 1991, 113, 9242.
- A. Rajca, S. Rajca, "Alkyl-Substituted Schlenk Hydrocarbon Diradicals with Triplet and Singlet Ground States in Frozen Solutions", J. Chem. Soc. Perkin 2, 1998, 1077.
- A. Rajca, S. Utamapanya, "π-Conjugated Systems with Unique Electronic Structure: A Case of "Planarized" 1,3-Connected Polyarylmethyl carbodianion and Stable Triplet Hydrocarbon Diradical", J. Org. Chem., 1992, 57, 1760.
Since these diradicals were quite stable at room temperature, a procedure for obtaining the solid diradicals was developed. We had in mind that isolation of these radicals as solid would allow for magnetic characterization using SQUID (Superconducting QUantum Interference Device). At that time, a SQUID was not available at KSU, so we had to find an instrument at nearby locations. With the help of a friend, Dr. Anton Nazareth, a postdoc at the Physics Department at the University of Nebraska in Lincoln (UNL), an arrangement was made for using the SQUID in Professor Sy-Hwang Liou's lab at the rate of $150/day.
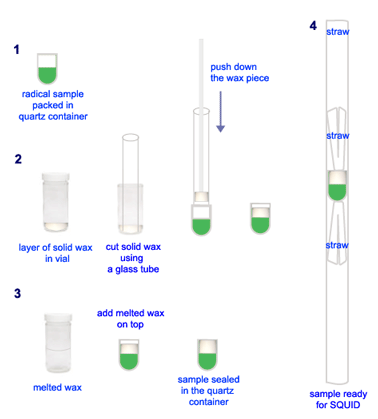
The momentous challenge was in preparation of samples for SQUID measurement. Gelatin capsule was commonly used as sample container for powdered sample (see MPMS Application Note 1014-201). However, the polyradical solid was air sensitive, so we probed different methods. At the end, we came up with the idea of sealing the powder sample inside a small quartz container with wax. Sealing of sample with wax required the use of melted wax. Exposing polyradical sample to above room temperature was the condition we ought to avoid. So, we worked out the procedure to prevent that [Technical Note 1].
Later on this procedure was modified to use an EPR tube [Technical Note 2] and finally to use a special homemade quartz tube [Technical Note 3].
To see more structures of diradicals prepared by our group, follow this
link.
Technical Notes

UNDER CONSTRUCTION
Quantum Design MPMS Application Note 1014-201: Sample Mounting Considerations.
Note 1: Preparation of Solid Samples for SQUID
Solid diradicals were prepared in a double-tube recrystallizer (H-Schlenk tube) equipped with high-vacuum stopcocks and ultra fine glass frit. Dianion was generated (from diether/THF/Li reaction) in one tube, and then filtered through the frit into another tube, where oxidation reaction (with I2) take place, to give green solid diradical. Following removal of THF under vacuum, the solid was washed with deoxygenated MeOH, then dried under vacuum overnight. The sample was stored in the refrigerator inside glovebox.
For SQUID experiment, the solid sample was packed into a small quartz container made from an EPR tube. First, the quartz piece was weighted on analytical balance. Then, loaded the solid sample and packed it using a glass rod. Record the sample weight. Put small amount solid wax in a vial, melted it on hot plate and let it to cool down and form a solid layer of about 2-3 mm thick. Use a glass tubing, with the same diameter as that of the quartz container, to cut out a piece of solid wax. Bring the solid wax in the glass tube over onto the solid diradical in the quartz container; pushed the wax piece out onto the sample. Finally, added a thin layer of melted wax on top. The sample was sealed tightly inside the quartz container! Next step, mounted the sample inside a drinking straw by using two small straw pieces to trap the sample container inside. The whole assembly was placed in a glass tube capped with a septum. The sample was taken out from the glovebox just before the trip to UNL. The samples were always prepared in advance, the day before the SQUID schedule.
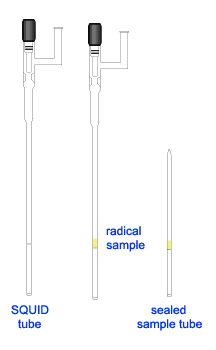
Note 2: Preparation of Solid Samples for SQUID: Modification
With the success in preparation of frozen polyradical for SQUID experiment, we imitate the sample configuration for the solid sample, i.e. having the solid sample band in between wax layers inside an EPR tube. The sample was prepared as follows.

Inside the glovebox, prepare melted wax in a vial. To prevent solidification of wax, slightly warmed up an EPR tube before adding the melted wax into the tube. Let the wax cool down and used a flat ended glass rod (or a Teflon ended metal rod) to pack down the wax, making a flat top layer. Weigh the EPR tube with wax before adding the solid diradical into the tube. It was helpful to use a long stem glass funnel, which reached down to a few millimeters above the wax layer. This prevent the solid sample from sticking on top part of the EPR tube, which would lead to inaccurate sample weight as well as to contaminate the top layer wax. Pack down the solid sample, forming a sample band of about 4 mm thick. Weight the EPR tube (with wax and sample). As described in Note1, prepare a thin layer of solid wax and inserted it onto the sample. Add melted wax to finish up the top layer.
Note 3: Preparation of Solid Samples for SQUID: Modification
... more detail coming soon!
|

